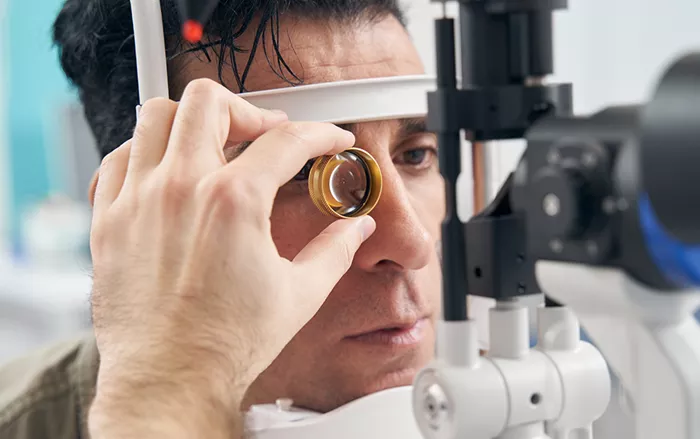
After a months-long investigation, the European Medicines Agency’s (EMA) Pharmacovigilance Risk Assessment Committee (PRAC) has confirmed a possible link between semaglutide use and a rare eye disorder called non-arteritic anterior ischemic optic neuropathy (NAION). This condition occurs when blood flow to the optic nerve is reduced, potentially leading to vision problems.
The risk of developing NAION while taking semaglutide is very low, estimated to affect up to one in 10,000 patients. However, the EMA noted that people with diabetes using semaglutide have twice the risk of developing this condition compared to those not taking the drug.
In response, the EMA has requested updates to the product information for semaglutide-containing medicines. These updates will include clear warnings about the potential risk of NAION. The European Commission will make the final decision on these changes.
Semaglutide, produced by Danish company Novo Nordisk, has seen a surge in use in recent years. Originally developed to manage type 2 diabetes, the drug has also gained popularity for weight loss. This widespread use beyond approved medical indications has raised concerns about long-term safety.
The EMA does not recommend stopping semaglutide treatment but urges doctors and patients to be more vigilant. Any sudden visual symptoms, such as blurred or lost vision, should be reported immediately and treated as a medical emergency.
Related Topics:
Sara Arfeen Khan Fitness Transformation Is Monday Motivation We Need
Pickup USA Fitness Basketball Club Coming to Sicklerville
Cameron Schmidt Excels at 2025 NHL Combine, Wins Third Gold with Team Canada